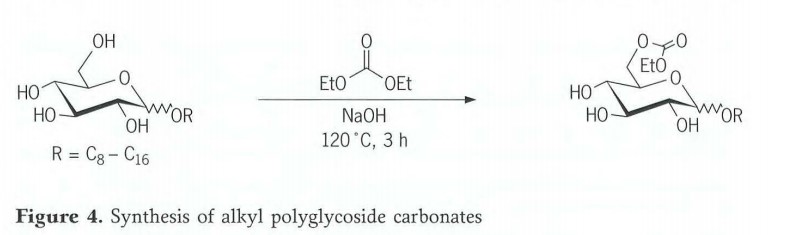
Hoʻohui ʻia o nā carbonates Alkyl polyglycoside
Ua hoʻomākaukau ʻia nā carbonates alkyl polyglycoside e ka transesterification o nā alkyl monoglycosides me diethyl carbonate (Figure 4). No ka hui pono ʻana o nā mea hoʻoheheʻe, ua ʻike ʻia he mea maikaʻi ke hoʻohana ʻana i ka diethyl carbonate i ʻoi aku ka maikaʻi i lilo ia i mea transesterification a me ka solvent. 2Mole-% o ka 50% sodium hydroxide solution e hoʻohui i ka dropwise i kēia hui me ka hoʻouluʻana ma kahi o 120 ℃. Ma hope o 3hours ma lalo o ka reflux, uaʻaeʻia ka huiʻana e hoʻomaha a hiki i 80 ℃ a neutralized me 85% phosphoric acid. Hoʻopau ʻia ka diethyl carbonate i loko o ka vacuo. Ma lalo o kēia mau ʻano hopena, ʻoi aku ka maikaʻi o ka esterified hoʻokahi pūʻulu hydroxyl. ʻO ka ratio o ke koena educt i nā huahana ma 1:2.5:1(monoglycoside: Monocarbonate:Polycarbonate).
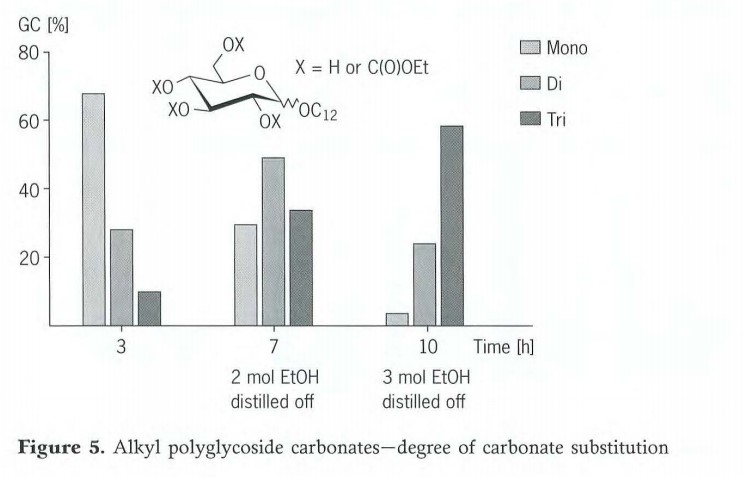
Ma waho aʻe o ka monocarbonate, hoʻokumu ʻia nā huahana me kahi kiʻekiʻe o ka hoʻololi ʻana i kēia pane. Hiki ke hoʻomalu ʻia ke degere o ka hoʻohui carbonate e ka hoʻokele akamai o ka hopena. No kahi C12 monoglycoside, loaʻa ka mahele o ka mono-, di- a me tricarbonate o 7:3:1 ma lalo o nā ʻano hopena i wehewehe ʻia (Figure 5). Inā hoʻonui ʻia ka manawa pane i 7 hola a inā hoʻopau ʻia nā 2moles o ka ethanol i kēlā manawa, ʻo ka huahana nui C.12 monoglycoside dicarbonate. Inā hoʻonui ʻia i 10 mau hola a hoʻopau ʻia nā 3moles o ka ethanol, ʻo ka huahana nui loa i loaʻa ka tricarbonate. Hiki ke hoʻololi maʻalahi ka degere o ka hoʻohui carbonate a no laila ke koena hydrophilic/lipophilic o ka pūhui alkyl polyglycoside ma o ka hoʻololi ʻana o ka manawa pane a me ka leo distillate.
Ka manawa hoʻouna: Mar-22-2021