Ma waho aʻe o ka ʻenehana, ʻo ka synthesis o glycosides i makemake mau i ka ʻepekema, no ka mea, he hopena maʻamau ia i ke ʻano. ʻO nā pepa hou a Schmidt a me Toshima a me Tatsuta, a me nā ʻōlelo kuhikuhi he nui i ʻōlelo ʻia ma laila, ua ʻōlelo ʻia e pili ana i kahi ākea o nā mea hiki.
I ka synthesis o glycosides, ua hui pū ʻia nā ʻāpana nui-sugar me kahi nucleophiles, e like me nā alcohols, carbohydrates, a i ʻole nā proteins, inā he hopena koho me kekahi o nā pūʻulu hydroxyl o ka carbohydrate i koi ʻia, pono e pale ʻia nā hana ʻē aʻe a pau i ka hana mua. Ma ke kumu, nā kaʻina hana enzymatic a i ʻole microbial, ma muli o kā lākou koho, hiki ke hoʻololi i ka pale kemika paʻakikī a me nā ʻanuʻu deprotection e koho mai nā glycosides i nā wahi. Eia nō naʻe, ma muli o ka mōʻaukala lōʻihi o nā alkyl glycosides, ʻaʻole i aʻo nui ʻia ka noi ʻana o nā enzymes i ka synthesis o glycosides.
Ma muli o ka hiki o nā ʻōnaehana enzyme kūpono a me nā kumukūʻai kiʻekiʻe, ʻaʻole mākaukau ka synthesis enzymatic o alkyl polyglycosides e hoʻonui i ka pae ʻoihana, a ʻoi aku ka maikaʻi o nā ʻano kemika.
I ka makahiki 1870, ua hōʻike ʻo MAcolley i ka synthesis o "acetochlorhydrose" (1, figure2) ma ka hopena o ka dextrose (glucose) me ka acetyl chloride, ka mea i alakaʻi i ka mōʻaukala o nā ala synthesis glycoside.
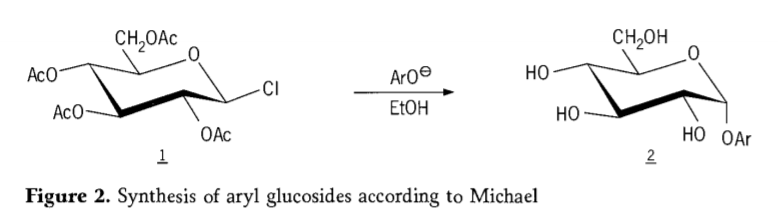
Ua ʻike ʻia ʻo Tetra-0-acetyl-glucopyranosyl halides(acetohaloglucoses) he mau kikowaena kūpono no ka hoʻohui stereoselective o nā alkyl glucosides maʻemaʻe. I ka makahiki 1879, ua kūleʻa ʻo Arthur Michael i ka hoʻomākaukau ʻana i nā aril glycosides mai nā mea waena a me nā phenolates a Colley. (Aro-, Kii 2).
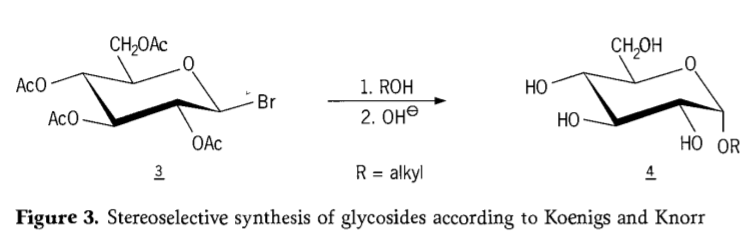
I ka makahiki 1901, ua hoʻokomo ʻia ʻo Michael i kahi ākea o nā carbohydrates a me nā aglycons hydroxylic, i ka wā i hoʻokomo ai ʻo W.Koenigs a me E.Knorr i kā lākou hoʻomaikaʻi ʻana i ke kaʻina hana stereoselective glycosidation (Figure 3). ʻO ka hopena e pili ana i kahi pani SN2 ma ke kalapona anomeric a hele stereoselectively me ka hoʻohuli ʻana o ka hoʻonohonoho ʻana, e hana ana no ka laʻana ka α-glucoside 4 mai ka β-anomer o ka aceobromoglucose intermediate 3. ʻO ka Koenigs-Knorr synthesis e hana ʻia ma ke alo o nā mea hoʻolaha kālā a mercury paha.
I ka makahiki 1893, ua noi ʻo Emil Fischer i kahi ala ʻokoʻa loa i ka synthesis o nā alkyl glucosides. Ua ʻike nui ʻia kēia kaʻina hana ʻo "Fischer glycosidation" a loaʻa i ka hopena acid-catalyzed o glycoses me nā waiʻona. E komo pū kekahi moʻolelo mōʻaukala i ka hoʻāʻo mua ʻana a A.Gautier i ka makahiki 1874, e hoʻohuli i ka dextrose me ka ethanol anhydrous i mua o ka waika hydrochloric. Ma muli o kahi loiloi elemental hoʻopunipuni, ua manaʻo ʻo Gautier ua loaʻa iā ia kahi "diglucose". Ua hōʻike ʻo Fischer ma hope mai ʻo ka "diglucose" a Gautier ʻo ia ka ethyl glucoside (Figure 4).
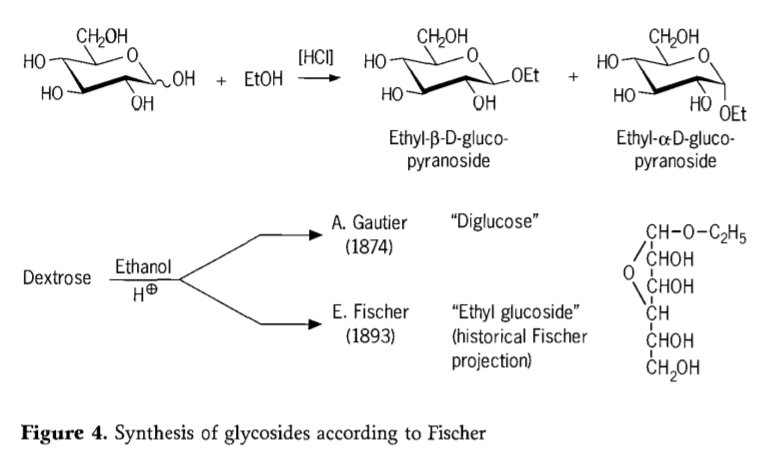
Ua wehewehe pololei ʻo Fischer i ke ʻano o ka ethyl glucoside, e like me ka mea i ʻike ʻia mai ka moʻolelo furanosidic formula i manaʻo ʻia. ʻO ka ʻoiaʻiʻo, paʻakikī nā huahana glycosidation Fischer, ʻo ka hapa nui o nā hui pū ʻana o nā α/β-anomers a me nā isomers pyranoside/furanoside i hui pū ʻia me nā oligomers glycoside i hoʻopili ʻia.
No laila, ʻaʻole maʻalahi ka hoʻokaʻawale ʻana i kēlā me kēia ʻano molekala mai nā hui ʻana o Fischer, kahi pilikia koʻikoʻi i ka wā ma mua. Ma hope o ka hoʻomaikaʻi ʻana i kēia ʻano hana synthesis, ua ʻae ʻo Fischer i ka synthesis Koenigs-Knorr no kāna mau noiʻi. I ka hoʻohana ʻana i kēia kaʻina hana, ʻo E.Fischer lāua ʻo B.Helferich ka mea mua i hōʻike i ka synthesis o kahi alkyl glucoside kaulahao lōʻihi e hōʻike ana i nā waiwai surfactant ma 1911.
I ka makahiki 1893, ua ʻike pono ʻo Fischer i nā waiwai koʻikoʻi o nā alkyl glycosides, e like me ko lākou kūpaʻa kiʻekiʻe e pili ana i ka oxidation a me ka hydrolysis, ʻoi aku hoʻi i ka media alkaline ikaika. He waiwai nā hiʻohiʻona ʻelua no nā alkyl polyglycosides i nā noi surfactant.
Ke hoʻomau nei ka noiʻi e pili ana i ka hopena glycosidation a ua hoʻomohala ʻia kekahi mau ala hoihoi i nā glycosides i ka wā i hala. Ua hōʻuluʻulu ʻia kekahi o nā kaʻina hana no ka synthesis o glycosides ma ka Figure 5.
Ma ka laulā, hiki ke hoʻokaʻawale ʻia nā kaʻina hana glycosidation kemika i nā kaʻina e alakaʻi ana i ka equilibria oligomer paʻakikī i ka hoʻololi glycosyl acid-catalysed.
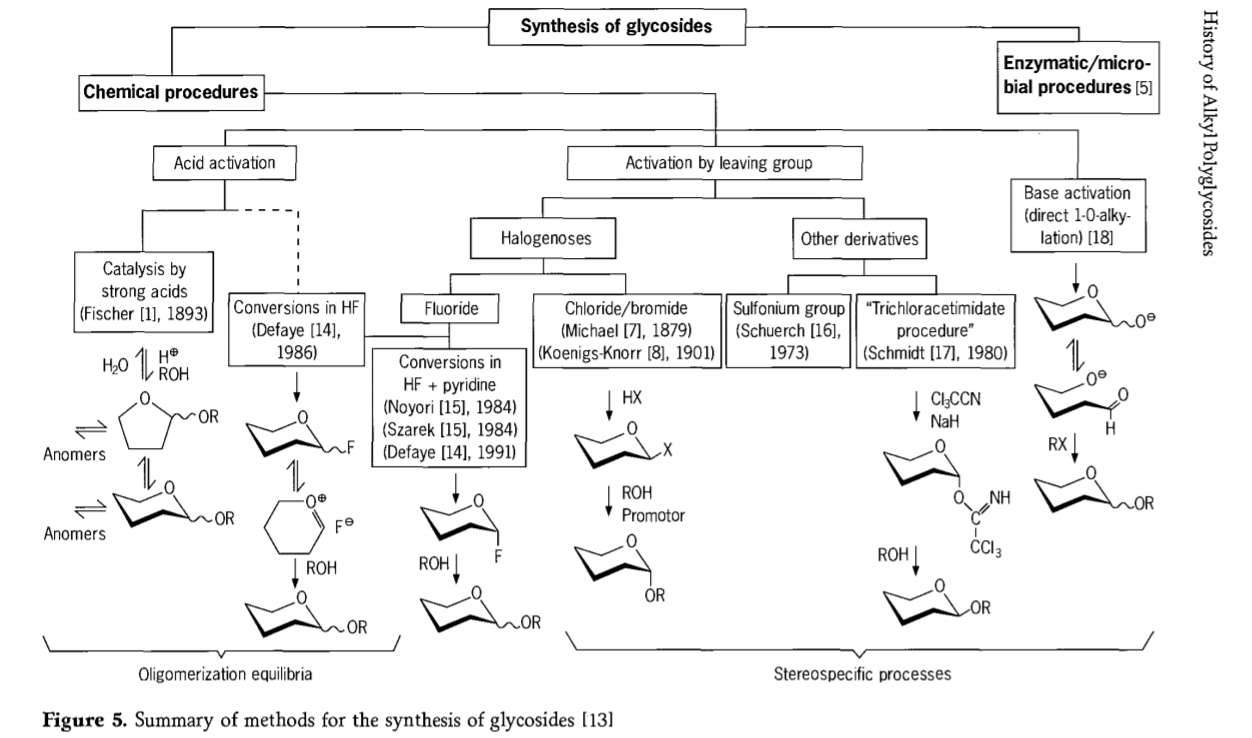
ʻO nā pane i nā substrates carbohydrate i hoʻoikaika pono ʻia (Fischer glycosidic reactions and hydrogen fluoride(HF) reactions with unprotected carbohydrate molekula) and kinetics controlled, irreversible, and most stereotaxic substitution reactions. Hiki i ke ʻano ʻelua o ke kaʻina hana ke alakaʻi i ka hoʻokumu ʻana o kēlā me kēia ʻano ma mua o ka hoʻohuihui paʻakikī o nā hopena, ʻoi aku ka hoʻohui ʻia me nā ʻenehana hui mālama. Hiki i nā kalapona ke waiho i nā hui ma ke kalapona ectopic, e like me nā mea halogen, sulfonyls, a i ʻole nā hui trichloroacetimidate, a i ʻole e hoʻāla ʻia e nā kumu ma mua o ka hoʻololi ʻana i nā esters triflate.
I ka hihia kūikawā o nā glycosidations i loko o ka hydrogen fluoride a i ʻole i loko o ka hui ʻana o ka hydrogen fluoride a me ka pyridine (pyridinium poly [hydrogen fluoride]), ua hoʻokumu ʻia nā glycosyl fluoride ma ka wahi a ua hoʻololi mālie ʻia i glycosides, no ka laʻana me nā waiʻona. Ua hōʻike ʻia ʻo Hydrogen fluoride he mea hoʻoikaika ikaika, nondegrading reaction medium; ʻIke ʻia ka equilibrium auto condensation(oligomerization) e like me ke kaʻina hana Fischer, ʻoiai ʻokoʻa paha ke ʻano hana.
ʻO nā alkyl glycosides maʻemaʻe maʻemaʻe he kūpono wale no nā noi kūikawā. No ka laʻana, ua hoʻohana maikaʻi ʻia nā alkyl glycosides i ka noiʻi biochemical no ka crystallization o nā protein membrane, e like me ka crystallization ʻekolu o ka porin a me ka bacteriorhodopsin i mua o ka octyl β-D-glucopyranoside (nā hoʻokolohua hou aʻe e pili ana i kēia hana ke alakaʻi i ka makana no ka Nobel no Deisenhofer, Huber a me Michel ma 1988).
I ka wā o ka hoʻomohala ʻana o ka polyglycosides alkyl, ua hoʻohana ʻia nā ʻano stereoselective ma ka pālākiō keʻena e synthesize i nā ʻano mea hoʻohālike like ʻole a noʻonoʻo i kā lākou mau waiwai physicochemical, ma muli o ko lākou paʻakikī, ka paʻakikī o nā waena a me ka nui a me ke ʻano koʻikoʻi o nā mea hoʻoheheʻe kaʻina hana, syntheses o ka ʻano Koenigs-Knorr a me nā pilikia ʻenehana pale a me nā ʻenehana. ʻO nā kaʻina hana ʻano Fischer ʻaʻole paʻakikī a maʻalahi hoʻi e hoʻokō ma ka pālākiō ʻoihana a no laila, ʻo ia ke ala i makemake ʻia no ka hana ʻana i nā alkyl polyglycosides ma kahi nui.
Ka manawa hoʻouna: Sep-12-2020





